Kips Bay Medical is committed to performing clinical studies to demonstrate long-term outcome results for the eSVS Mesh technology. We are currently involved in an FDA-mandated feasability study and are sponsoring ongoing post-market studies in Europe.

PRE-CLINICAL TESTING >
The eSVS Mesh has been studied in a number of animal models to confirm performance expectations. These studies showed a statistically significant inhibition of intimal hyperplasia when the eSVS Mesh was used in CABG procedures.
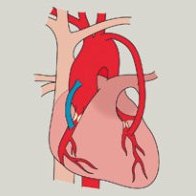
CLINICAL TRIALS >
With the U.S. Food and Drug Administration (FDA) approval to begin a feasibility trial, we are working with some of the leading cardiac research centers in the United States and Europe.
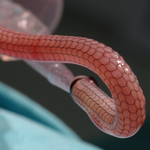
INSTRUCTIONS FOR USE >
The eSVS Mesh is a highly flexible, semi-compliant, kink-resistant extravascular tubular prosthesis made of knitted nickel/titanium (nitinol) wire. The eSVS Mesh is designed for maintaining the patency of autologous vein grafts (i.e. saphenous vein) used in coronary artery bypass grafting (CABG) surgery.
RESOURCE GALLERY >
Our online media gallery features product animations and surgical videos demonstrating the preparation and deployment of the eSVS Mesh.


